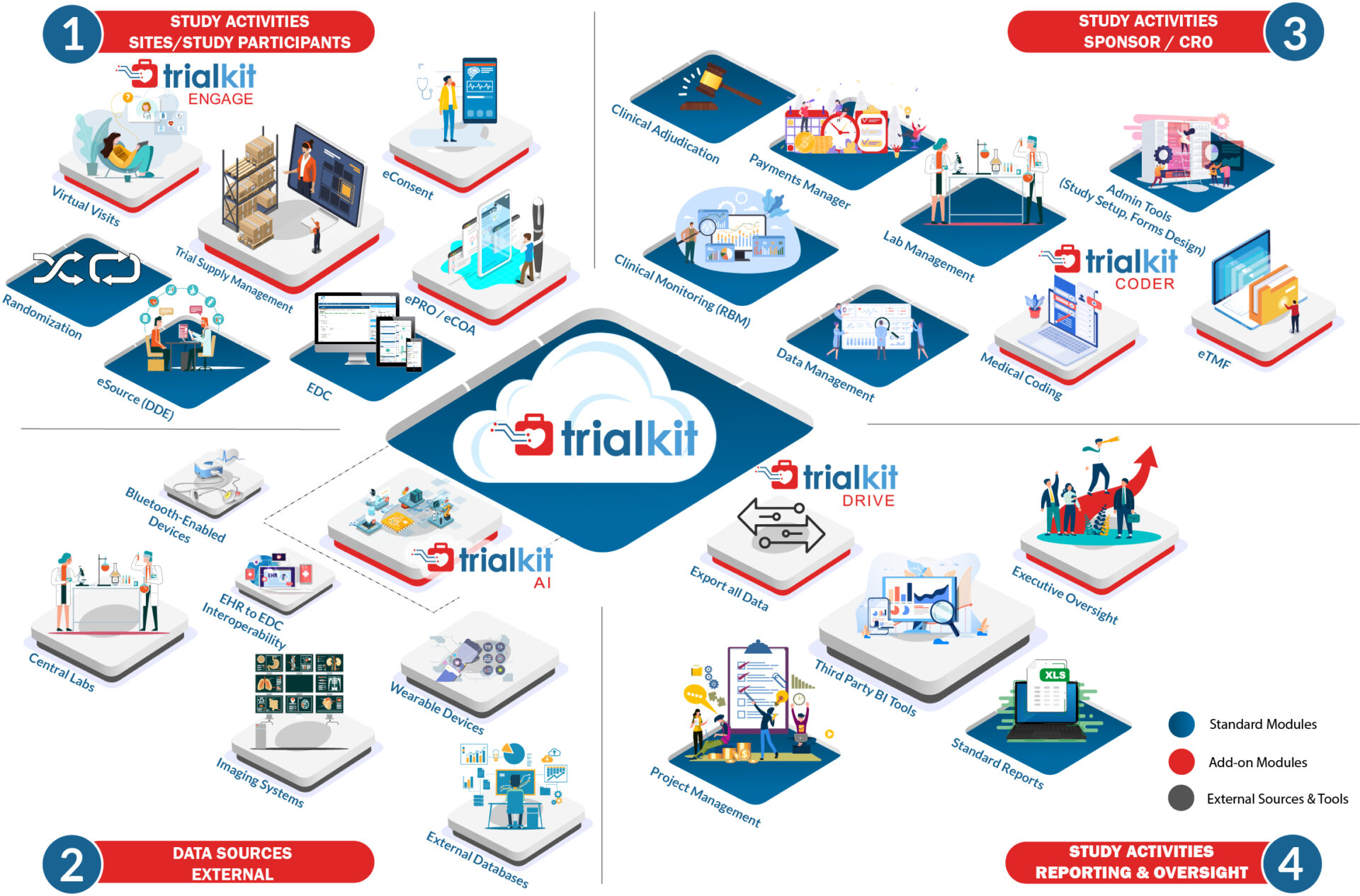
The only truly unified self-service solution for clinical trials and non-interventional studies
Run clinical trials and registry studies with more flexibility and control than ever.



Ready to hit the ground running with your clinical trials, study after study?
Our unified, ultra-adaptable Software-as-a-Service platform, TrialKit, makes it easy for you to design and execute clinical trials. Any study design, size, or therapeutic area.
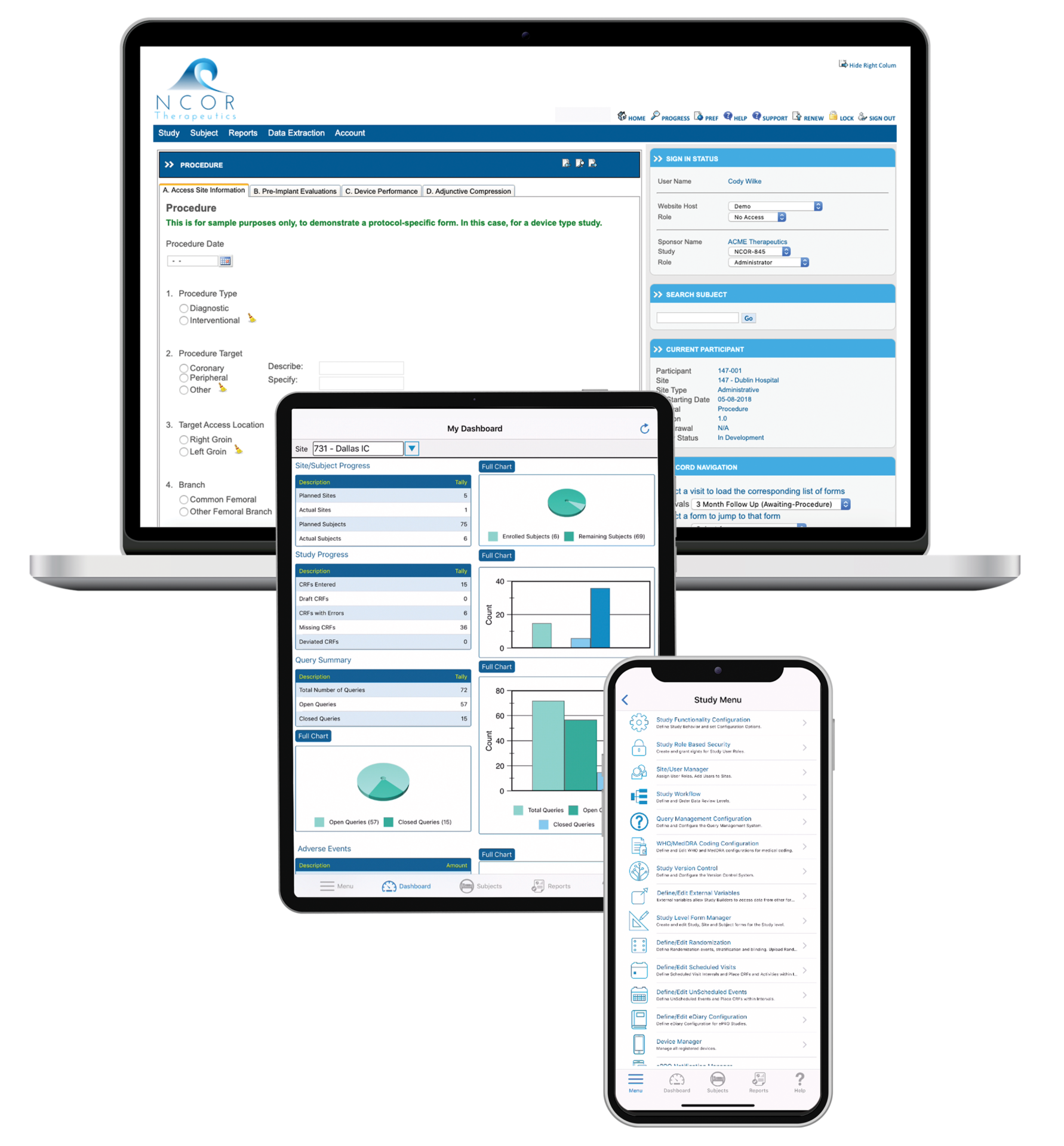
Fully available on the web and on a native mobile app, TrialKit powers all study designs, all therapeutic areas, and all levels of complexity. From EDC to advanced reporting and analytics, TrialKit is the simple solution for complex clinical trials.
TrialKit EDC
End-to-end, cloud-based solution that enables interchangeable use of browser-based and mobile app platforms.
Read more →TrialKit eSource (Direct Data Capture)
Capture source data directly from sites who are able to enter data directly from a smartphone or tablet, eliminating SDV.
Read more →TrialKit eCOA | ePRO
Leverage mobile ePRO/eCOA for direct electronic patient-reported outcomes through a BYOD approach.
Read more →TrialKit eConsent
Use powerful automation to electronically consent and re-consent participants, if required, via the web and/or mobile app.
Read more →TrialKit Electronic Trial Master File (eTMF) System
Online folders for creation of storing, accessing, and tracking essential documents for the submission of study documentation.
Read more →TrialKit RTSM or IRT
Inventory/Trial Supply Management module for web and mobile can be configured to randomize and track subjects, drug supply, devices and inventory throughout the course of a study.
Read more →TrialKit Payment Manager
Integrated site payments tracking and accrual based on clinical activities as entered and completed.
Read more →Data Reporting and Analytics with TrialKit Drive
Out-of-the-box dashboards, ad hoc reports, and full data exports for reporting and analysis available for all clinical data.
Read more →TrialKit Clinical Adjudication
Flexibility to configure and define the adjudication process and rules as well as to perform all adjudication tasks from the web and mobile app.
Read more →TrialKit Coder for Medical Coding
Automated medical coding functionality (for WHODrug and/or MedDRA dictionaries).
Read more →TrialKit AI for EHR to EDC Connectivity
First-of-its-kind technology using our proprietary machine-learning API to transform unstructured EHR or other data into usable data, connecting it to a structured database for your clinical trial data or other validated data.
Read more →TrialKit Engage for Televisits
An integrated video conferencing and scheduling solution accessible to study teams, site personnel, and study participants.
Read more →Clinical Trial Imaging | TrialKit PACS
Access, interact with, and even adjudicate clinical trial imaging from the same platform where you manage all other study data.
Read more →
Clinical Trial SaaS with a Capital ‘S’
From small biologics trials to concurrent Phase 3 pharmaceutical trials and massive registry studies, TrialKit gives you the freedom to capture and manage your clinical trial data your way—no programming required.
Here’s why we’re the most flexible clinical technology out there:
Flexibility to manage
Design it with your team, your way. Our drag-and-drop, click-and-configure design tool makes it easy to DIY.
Flexibility to scale
We’re the only eClinical platform that can run massive studies on multiple clouds with single sign-on (SSO).
Flexibility in study design
TrialKit offers the only mobile-native EDC that works for traditional, hybrid/decentralized, and fully remote trials.
Flexibility to rebrand
Make TrialKit your own with white label and custom URL options.
Flexibility in pricing
Choose from affordable study-by-study or enterprise-scale subscriptions.
Why Choose TrialKit for Your Clinical Trials?
Ultimate Control
Run studies the way you want, without waiting or relying on others (including us). TrialKit empowers you to collect, view, and manage your data from anywhere on any device.
Mobile First
Collect electronic clinical outcomes assessment (eCOA) data from patients remotely or on site. Use provisioned devices, a BYOD approach, or both. Our native mobile app simplifies remote study management and participation.
No Programming Required
Our drag-and-drop format lets you take a DIY approach to study build and modifications.
Unlimited Integration
Move data from wearables, sensors, apps, and electronic health records (EHRs) without expensive workflow changes. Our open API architecture allows you to safely link any external devices and software to TrialKit, eliminating duplicative data entry.
Minimal Training
Get up to speed in hours, not weeks. With no programming or software development required, it takes little training to learn TrialKit. 24/7 tech support and a comprehensive knowledge base are here to help if you get stuck.
Leading medtech, pharmaceutical, and biotech sponsors and CROs have trusted their studies to TrialKit since 2010.
1000+
Customers
8,000+
Studies
7
Continents
We love the flexibility to modify live studies, reuse CRFs (which hastens development time for each successive study) and create stacked edit checks and conditional actions. The transparent pricing structure removes the high costs associated with launching and running studies.
Clinical Data Manager

I’ve gotten the cleanest data that I’ve ever worked with [with TrialKit].
Research Coordinator

We rely on TrialKit to document the collection of dried blood samples and upload data from users’ mobile devices to secure storage in the cloud. Collaborating with the experienced TrialKit team has been a very cost-effective method to rapidly design and test a capable and polished product.
CEO

I have built databases using SAS and Oracle Clinical, and have used many other systems. TrialKit is by far my favorite. The customer support is better than anything I’ve seen, literally, they are always just a phone call away and go above and beyond to make sure their clients are happy. TrialKit is always seeking to improve their products and are always ready and willing to hear new ideas, and often incorporate them. I love this product and support staff.
Clinical Data Manager

The customer support provided by the TrialKit team was exceptional. They are responsive, knowledgeable, and always ready to assist with any questions or concerns. Knowing I have a dedicated support team backing me up was reassuring. Overall, TrialKit has simplified and automated my trial management process, saving me time and effort while delivering excellent results. It’s a flexible tool for a start-up or larger trial, and I highly recommend TrialKit to anyone looking for an EDC to run their study. Thank you, Crucial Data Solutions, for being a great partner and support.
Head of Clinical Operations

TrialKit is an affordable, flexible, easy to use, integrated system. It allows us to reduce study build times and helps us to deploy study databases in a more timely manner for our clients. The support staff is extremely responsive and knowledgeable, and they are always seeking ways to improve their products. We have a very collaborative working relationship with the team at CDS.
SVP Resourcing Operations